No Belt, no Strap and no Parachute
Drug shortages reported by local Authorities in Europe and in the USA are growing and life supporting equipment are becoming scarce. Why are our life sciences supply chain so vulnerable? For years, the industry has worked on business continuity plans, why are we suddenly so exposed to risks which seem not to have merely been prognosticated. What are the root causes of this cataclysm?
This paper highlights that the risk management fundamentals have not changed. The industry needs to continue applying the traditional methodology. However, a disruptive approach should be taken when quantifying the impacts of each risk. We also highlight how to protect your business operations and supply chain and make them more resistant to shocks.
| Risk cautious versus cost cautious | For many years the pharma supply chain leadership teams have been cost cautious when delivering their mitigation action plans. Shareholders’ pressure to publish higher margin was a key consideration when approving required CAPEX and OPEX plans to de-risk the global supply chain. Identifying the risks is not the issue here. In all the cases Supply Chain Operations has analysed over the years, it was always done meticulously and with great care. The teams were looking at a wide range of factors, from pure supply chain risks to emerging ones to adverse unpredictable events such as fire or earthquake. Most of the potential disruptions factors and cases were covered. But when it came to the risk mitigation actions no importance was given to invest in order to reduce business impact of major risks that have not happened yet, or which have a low probability of occurring
In other words, budget constraints as well as the perceived lack of danger coming from low probability have always prevailed. For instance, for a pharma company, manufacturing the API (Active Pharmaceutical Ingredient) in one production plant in one geography and at full capacity enables to report to senior management attractive cost savings. Was de-risking the end-to-end supply chain really fully taken into account?Many life sciences companies took the risk to manufacture essential components of a registered drug in one location. Now we see the impact on the daily/weekly drug shortage reports issued by local Authorities in Europe, in the USA and in other countries. The current pandemic crisis is stressing even more the sourcing of key materials across the globe and putting patient at higher risk of getting their medicinal product on-time and in-full. De risking the supply chain should be performed keeping in mind the only real objective: “The Patient” and its health condition, nothing else. |
|||
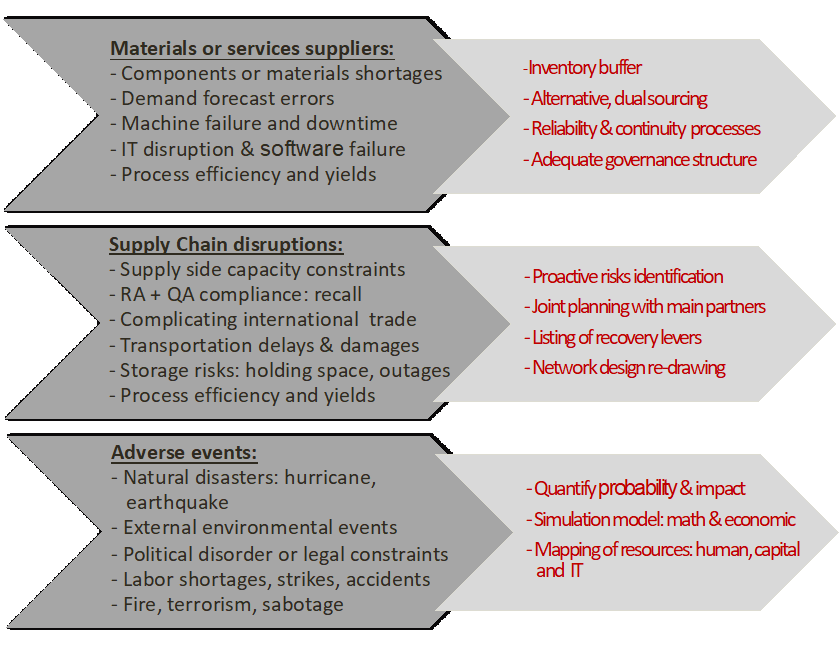
| Single point of failure | The background being set, let’s look forward. We would like to highlight two key aspects to focus on in order to de-risk your global supply chain: single point of failure and readiness to start.International or even intercontinental Supply chains are very complex and have always several single points of failure like, single sourcing of components, single manufacturing plant, single distribution center, single testing sides, single approved regulatory source or simply single dependency on a system or an operational process.
More attention should be given to identify single points of failure which should be distinguished between internal weaknesses and external vulnerabilities. The most common exposures can be classified as follows:
|
|||
| Recovery lead time and readiness to restart | Once the risk assessment has been thoroughly performed and validated by senior management, all our attention should be given to the recovery processes and lead-times. Planning for the restart of complex supply chain operations requires experience and know-how. Such exercise needs to be simulated, trained and adapted at least every year. What is true today when you draw your recovery plans will not apply next year when your business will have changed and grown. To guarantee that your plans are sound and ready for roll out the end-to-end value chain needs to be tested. Meaning involving some of your main customers, which is also a commercial approach to demonstrate your commitment to supply, and some of your suppliers. It will include evaluating their capability to cope with those supply chain risks and hick-ups which any business will be confronted several times in its lifetime.
Many Governments are now, during the crisis, considering to re-localize manufacturing of key components to guarantee timely and qualitative supply of strategic products even if production costs maybe be slightly higher. If your operations can restart and get back to normal quicker than your competition you definitely have a business advantage which may be significant in terms of supply volume and business value. |
|||
|
Digital technologies to speed up recovery timelines |
Nowadays new supply chain technologies are certainly embedded in their company DNAs and they are dramatically improving visibility and support companies’ ability to resist most economical and operational shocks. The traditional linear supply chain model is transforming into digital supply networks and eco-systems, where functional silos are broken down and organizations become connected to their complete supply network to enable responsiveness, agile collaboration, resilience and sustainability.Advanced technologies, such as the artificial intelligence, the robotics and the Internet of Things, are also the key to success with outstanding recovery timelines. Whether it is an unpredictable event like COVID-19, or any type of major accidental disruptions, your supply chain organization has to be ready to deal with the unexpected.
De-risk your supply chain design by leveraging our proven methodology as well as our expert support. |
The success of your supply chain de-risking project is directly conditioned and linked to the quality and experience of the resources you bring onboard. We know that each project is unique with its own constraints and challenges. Having a backbone of genuine expertise in bio-pharmaceutical supply chain is a strong plus to minimize risk impact and likelihood.
Be successful in your project . . . contact us today.
Supply Chain Operations SA, based in Switzerland, is a specialized healthcare supply chain boutique consultancy firm created in 2011 to serve the bio-pharmaceutical and MedTech industry. We bring more than 120 years of end-to-end supply chain expertise to our valued customers.
Pierre-Yves Bridel has 25 years’ experience in supply chain and sourcing with Life Sciences and FMCG companies. He specialized in crisis management and digital transformation.
Mobile: +41 79 743 74 82
Email : pierre-yves.bridel@supplychainoperations.ch
Website: www.supplychainoperations.ch
Laurent Foetisch of Supply Chain Operations SA has extensive experience as a supply chain executive responsible for managing a global bio-pharmaceutical company in Switzerland for more than 30 years. Since 2011 Laurent has developed Supply Chain Operations SA with the objective to bring life sciences supply chain experience and knowledge to its customers.
Mobile: +41 79 205 23 32
Email: laurent.foetisch@supplychainoperations.ch
Website: www.supplychainoperations.ch
Supply Chain Operations SA, based in Switzerland, is a specialized healthcare supply chain boutique consultancy firm created in 2011 to serve the bio-pharmaceutical and MedTech industry. We bring more than 120 years of end-to-end supply chain expertise to our valued customers.
Pierre-Yves Bridel has 25 years’ experience in supply chain and sourcing with Life Sciences and FMCG companies. He specialized in crisis management and digital transformation.
Mobile: +41 79 743 74 82
Email : pierre-yves.bridel@supplychainoperations.ch
Website: www.supplychainoperations.ch
Laurent Foetisch of Supply Chain Operations SA has extensive experience as a supply chain executive responsible for managing a global bio-pharmaceutical company in Switzerland for more than 30 years. Since 2011 Laurent has developed Supply Chain Operations SA with the objective to bring life sciences supply chain experience and knowledge to its customers.
Mobile: +41 79 205 23 32
Email: laurent.foetisch@supplychainoperations.ch
Website: www.supplychainoperations.ch